Editor’s Note: This story was updated with the FDA’s approval of donanemab.
In a highly anticipated decision, the U.S. Food and Drug Administration (FDA) has approved the early-stage Alzheimer’s drug Kisunla (donanemab).
This makes it the second medicine, after Leqembi (lecanemab), that can slow the progression of early-stage Alzheimer’s disease by targeting the underlying processes in the brain that lead to cognitive decline.
An FDA advisory panel endorsed approval of the Eli Lilly drug last month.
Amyloid Antibodies Clear Brain Plaques Connected to Alzheimer’s
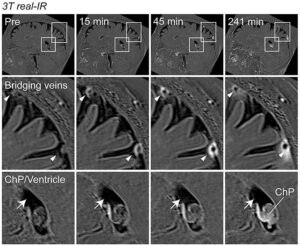
One safety concern with donanemab is the potential for people who take this drug and other amyloid antibodies to experience what’s known as ARIA, or amyloid-related imaging abnormalities. ARIA shows up on brain scans and can sometimes involve seizures or potentially life-threatening brain swelling or bleeding, although many people don’t experience symptoms.
Brain Bleeding and Swelling
In the late-stage donanemab trial, 37 percent of people on the drug experienced some type of ARIA with bleeding or swelling in the brain.
Like lecanemab, donanemab will require that patients get regular magnetic resonance imaging (MRI) scans to look for and address any bleeding or swelling in the brain.
“Most people aren’t going to get side effects that are symptomatic with ARIA,” says Lawrence Honig, MD, PhD, a neurologist at NewYork-Presbyterian/Columbia University Irving Medical Center in New York City. “The likelihood of symptomatic side effects that you feel and you notice or your family notices is less than 10 percent even for those most at risk.”
While the risk is slightly higher with donanemab than with lecanemab, some people may still prefer donanemab because it’s administered via monthly infusions instead of every two weeks with lecanemab, Dr. Honig says. However, at least initially, it may be easier for some people to access lecanemab because the drug has been on the market longer and supplies may be more readily available, Honig notes.







Post Comment