Long-acting HIV treatment demonstrates efficacy in people with challenges taking daily medicine as prescribed
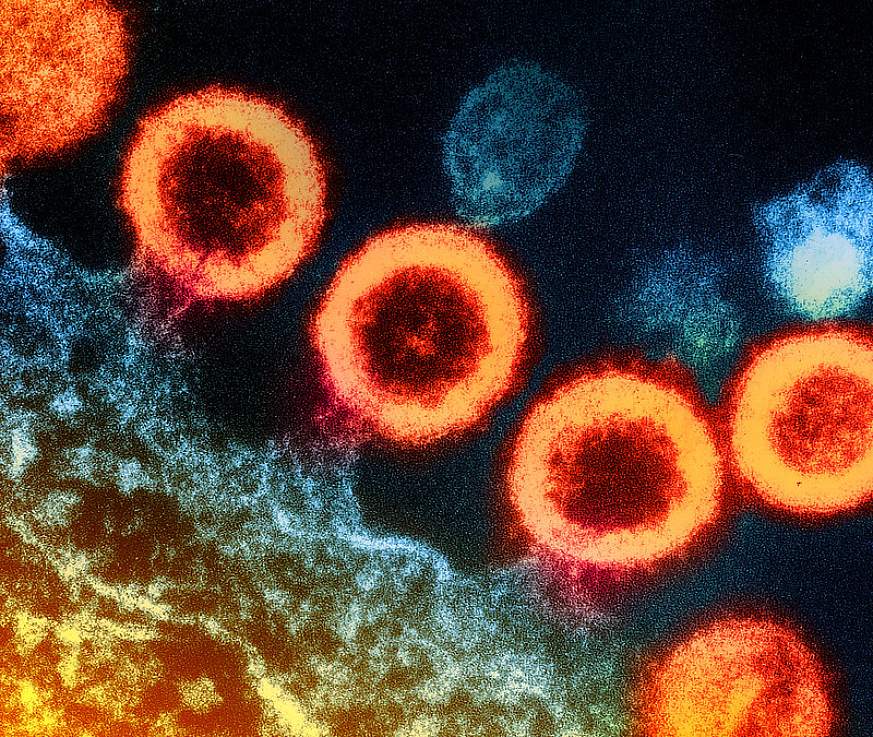
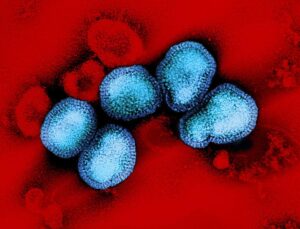
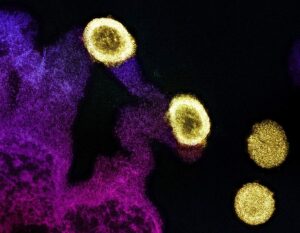
Transmission electron micrograph of HIV-1 virus particles (orange) replicating from the plasma membrane of an infected H9 T cell.NIAID
Long-acting antiretroviral therapy (ART) with cabotegravir and rilpivirine was superior in suppressing HIV replication compared to daily oral ART in people who had been unable to maintain viral suppression through an oral daily regimen, according to interim data from a randomized trial. Upon review of these findings, an independent Data and Safety Monitoring Board (DSMB) recommended halting randomization and inviting all eligible study participants to take long-acting ART. The study is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, in collaboration with the National Institute of Mental Health, the National Institute on Drug Abuse, ViiV Healthcare and ACTG, a global NIH-funded clinical trials network that designed and implemented the study. NIH accepted the DSMB’s recommendation.
About 70% of people with HIV in the United States who take oral ART achieve viral suppression, according to data from the Centers for Disease Control and Prevention. Viral suppression protects people with HIV from progressing to advanced disease, and prevents HIV transmission to others. In 2021, the Food and Drug Administration approved cabotegravir and rilpivirine as an ART regimen for people with a consistent history of viral suppression. NIAID launched a study in 2019 to understand how this regimen would work in people with previous challenges taking daily oral ART as prescribed and who had not consistently maintained viral suppression.
The study is ongoing across 31 sites in the United States including Puerto Rico. Participants were screened to ensure the HIV in their blood was not resistant to the study drugs and that they met other health and safety criteria. Once enrolled, they received comprehensive and incentivized adherence support while taking daily oral ART to achieve viral suppression. Participants who achieved viral suppression were then randomized to receive long-acting injectable ART every four weeks or to continue taking standard-of-care daily oral ART, with frequent health monitoring.
On February 12, the study DSMB conducted a planned review of interim data and found that long-acting cabotegravir and rilpivirine administered every four weeks had superior efficacy to daily oral ART. The DSMB recommended that all participants should have the opportunity to choose long-acting ART to ensure the best possible health outcomes in the study. Study participants are being notified of these findings and those currently taking daily oral ART will decide with their providers which ART method suits their needs best. All study participants will be monitored for another year.
ViiV Healthcare and Johnson & Johnson provided medications for this study. Detailed interim results will soon be shared through scientific channels.
NIH is grateful to the research sites and volunteers who participate in studies to improve HIV treatment.
NIAID conducts and supports research—at NIH, throughout the United States, and worldwide—to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health (NIH):
NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
NIH…Turning Discovery Into Health®





Post Comment