Long-acting HIV treatment benefits adults with barriers to daily pill taking and adolescents with suppressed HIV
NIH-funded research networks provide evidence on cabotegravir and rilpivirine in additional populations.
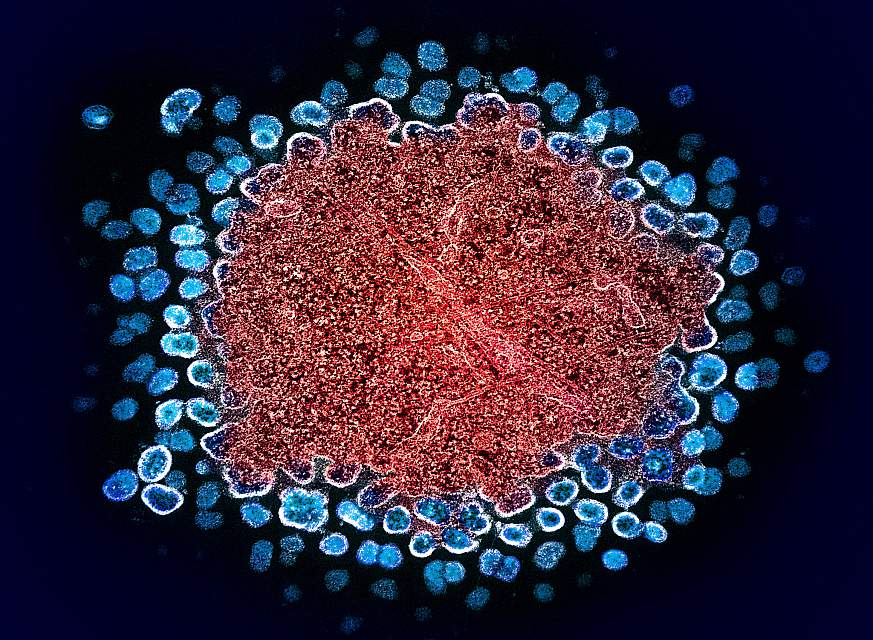
Colorized transmission electron micrograph of numerous HIV-1 virus particles (blue) replicating from a segment of a chronically infected H9 T cell (red).NIAID
Long-acting, injectable antiretroviral therapy (ART) suppressed HIV replication better than oral ART in people who had previously experienced challenges taking daily oral regimens and was found safe in adolescents with HIV viral suppression, according to two studies presented today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. Both studies were sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, in collaboration with other NIH institutes.
“The HIV community is just beginning to unpack the enormous potential of long-acting antiretroviral medications for HIV treatment and prevention, and we need population-specific data for everyone to benefit,” said NIAID Director Jeanne Marrazzo, M.D., M.P.H. “These findings open up new possibilities for millions of people with HIV, particularly those whose health suffers due to challenges of daily pill taking.”
NIH funded this research to build on the clinical trials that underpinned the Food and Drug Administration approval of long-acting cabotegravir and rilpivirine in 2021 for people with a consistent history of viral suppression. The studies were conducted in collaboration with ViiV Healthcare and Johnson & Johnson and intended to provide evidence on the use of the drug regimen in populations for whom limited data exist.
The first large clinical trial examined the effectiveness of long-acting injectable cabotegravir and rilpivirine coupled with support to help people who had experienced barriers to consistently taking daily oral ART. Participants were randomized to receive long-acting injectable ART every four weeks or standard-of-care daily oral ART. An interim analysis of data from 294 participants showed that the chance of experiencing unsuppressed HIV was 7% among people taking long-acting ART compared to 25% among those taking daily oral ART. The likelihood of discontinuing the assigned regimen due to adverse events or experiencing unsuppressed HIV was 10% among people taking long-acting ART compared to 26% among those taking daily ART. These findings were statistically significant. The study’s primary endpoint measured the probability that participants would experience unsuppressed HIV and discontinue their ART regimen for any reason, and while findings showed a favorable trend for long-acting ART compared to daily oral ART, the effect was not statistically significant based on the study’s stringent interim analysis criteria. The study is being implemented in the United States (including Puerto Rico) through ACTG, a global NIH-funded clinical trials network focused on HIV and other infectious diseases.
A subset of participants in both the long-acting and daily ART study arms remained unsuppressed on their assigned ART regimen, and two people with unsuppressed HIV in the long-acting arm were found to have HIV resistant to integrase inhibitors—cabotegravir’s drug class. More than half of participants taking long-acting ART experienced injection-site reactions, such as pain, redness, or swelling, and one discontinued use for that reason. On February 21, 2024, NIH announced that an independent Data and Safety Monitoring Board (DSMB) recommended halting randomization and offering all eligible study participants long-acting ART based on its observed superior viral suppression of HIV. NIH accepted the DSMB’s recommendation.
“This study shows long-acting technology is safe and effective among the people with HIV who stand to benefit most from its use,” said study chair Aadia Rana, M.D., professor of medicine at the University of Alabama-Birmingham (UAB) and senior scientist with the UAB Center for AIDS Research. “Offering an effective alternative for people who have struggled with taking daily ART could provide life-changing freedom from the stress of unsuppressed HIV.”
The study is being implemented in the United States (including Puerto Rico) through ACTG, a global NIH-funded clinical trials network focused on HIV and other infectious diseases, with support from NIAID, the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
A second clinical trial examined the safety of the same regimen administered every eight weeks in adolescents with HIV viral suppression. The study enrolled participants aged 12 to 17 in Botswana, South Africa, Thailand, Uganda and the United States. Of the 142 participants for whom data were available at 24 weeks, all remained virally suppressed and none experienced serious adverse events related to the drug regimen. Thirty-five percent of participants reported an injection-site reaction, the majority of which were mild and resolved within seven days. No one discontinued use of the regimen due to adverse events. As required by the study protocol, one participant discontinued study drugs due to an unintended pregnancy. They had a live birth with no complications. The drug concentrations of cabotegravir and rilpivirine in adolescents were comparable to levels generally observed in adults. These data support the use of cabotegravir and rilpivirine in virally suppressed adolescents, according to the authors.
“This is the first group of adolescents with HIV to have an alternative to daily pills for treatment, and the outcomes we observed have been very encouraging,” said study co-chair Aditya Gaur, M.D., director of the Division of HIV Medicine at St. Jude Children’s Research Hospital. “This study is part of the commitment to speed the evaluation of novel therapies for pediatric and adolescent populations, so that they have the opportunity to benefit as soon as possible.”
The study is being conducted through the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network with support from NIAID, NICHD, and NIMH.
NIH is grateful to the research sites and volunteers who participate in studies to improve HIV treatment.
For more information about the study of cabotegravir and rilpivirine in adults with barriers to daily pill taking, please see ClinicalTrials.gov using the identifier NCT03635788. For more information about the study of the regimen in virally suppressed adolescents, please use identifier NCT03497676.
NIAID conducts and supports research—at NIH, throughout the United States, and worldwide—to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health (NIH):
NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
NIH…Turning Discovery Into Health®
References:
Rana et al. Long-acting Injectable CAB/RPV is Superior to Oral ART in PWH with adherence challenges: ACTG A5359. Conference on Retroviruses and Opportunistic Infections in Denver, Colorado. Wednesday, March 6, 2024.
Gaur et al. Long-acting Cabotegravir Plus Rilpivirine in Adolescents with HIV: Week 24 IMPAACT 2017 (MOCHA) Study. Conference on Retroviruses and Opportunistic Infections in Denver, Colorado. Wednesday, March 6, 2024.






Post Comment